Welcome to the
Santerre Lab at Temple University!
We study the molecular mechanisms of HIV-Associated Neurocognitive Disorder (HAND), focusing on autophagy, lysosomal and mitochondrial dysfunctions
OUR RESEARCH
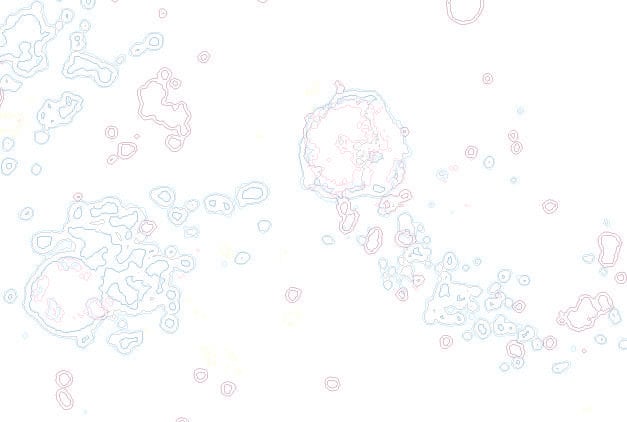
Our research focuses on how HIV-1 viral proteins disrupt neuronal function and drive neurodegenerative processes that contribute to HIV-associated neurocognitive disorders (HAND). A key area of interest is the autophagy and lysosomal system, where we investigate how HIV-1 impairs lysosomal function, a critical component in cellular waste processing and intracellular signaling. Beyond autophagy dysfunction, we also examine the virus's impact on mitochondrial health, as mitochondria are essential for energy production and cellular homeostasis.
To tackle these questions, among many techniques, we use live-cell confocal imaging. We also perform organellar omics studies to map dynamic changes in protein localization and signaling pathways affected by HIV-1. By identifying these pathways, we aim to uncover new therapeutic targets to mitigate HIV-induced neurodegeneration.
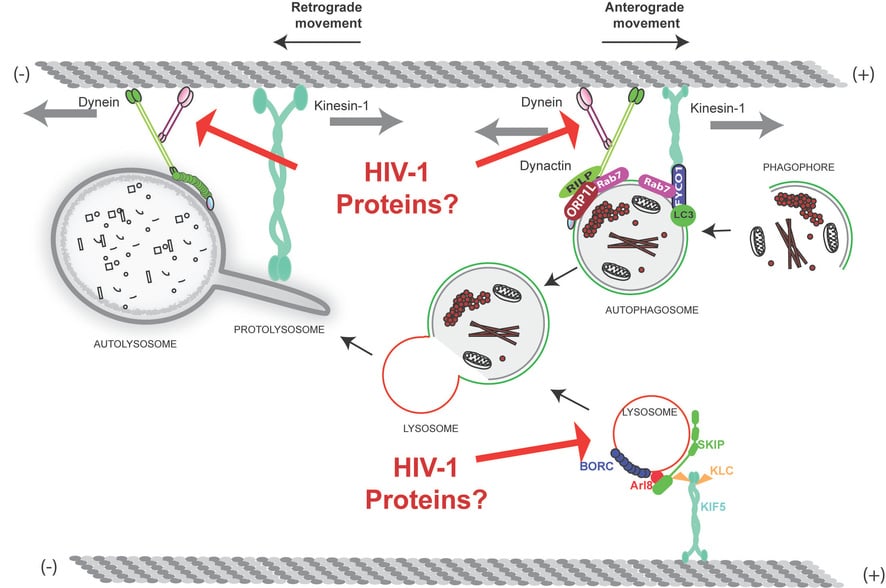
Live-Cell Confocal Microscopy
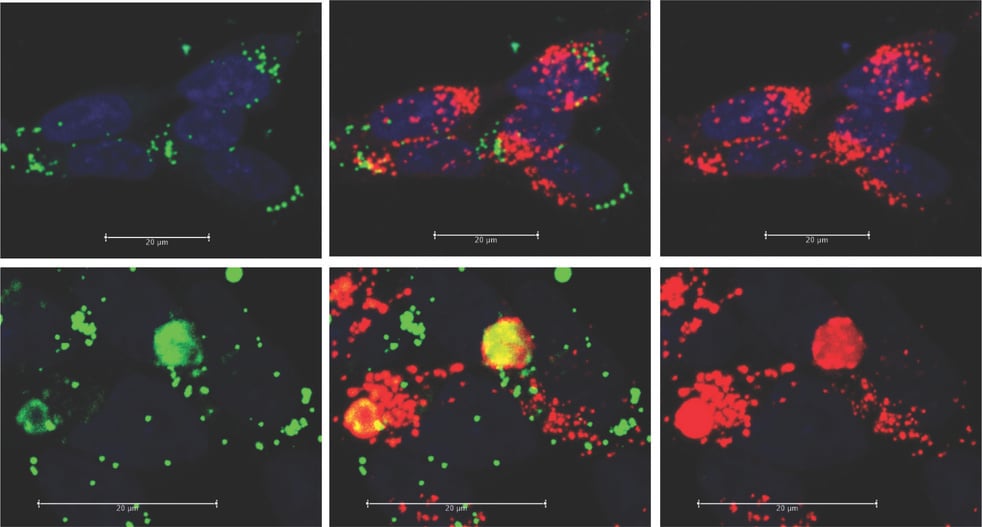
Live-cell confocal microscopy is a powerful tool for studying autophagy, lysosomes, and mitochondria. This technique enables the tracking of organelle dynamics, autophagosome formation, lysosomal acidification, and mitochondrial /lysosomal health, offering critical spatial and temporal resolution to observe how these organelles respond to various stimuli or stressors in a live environment.
Organellar omics
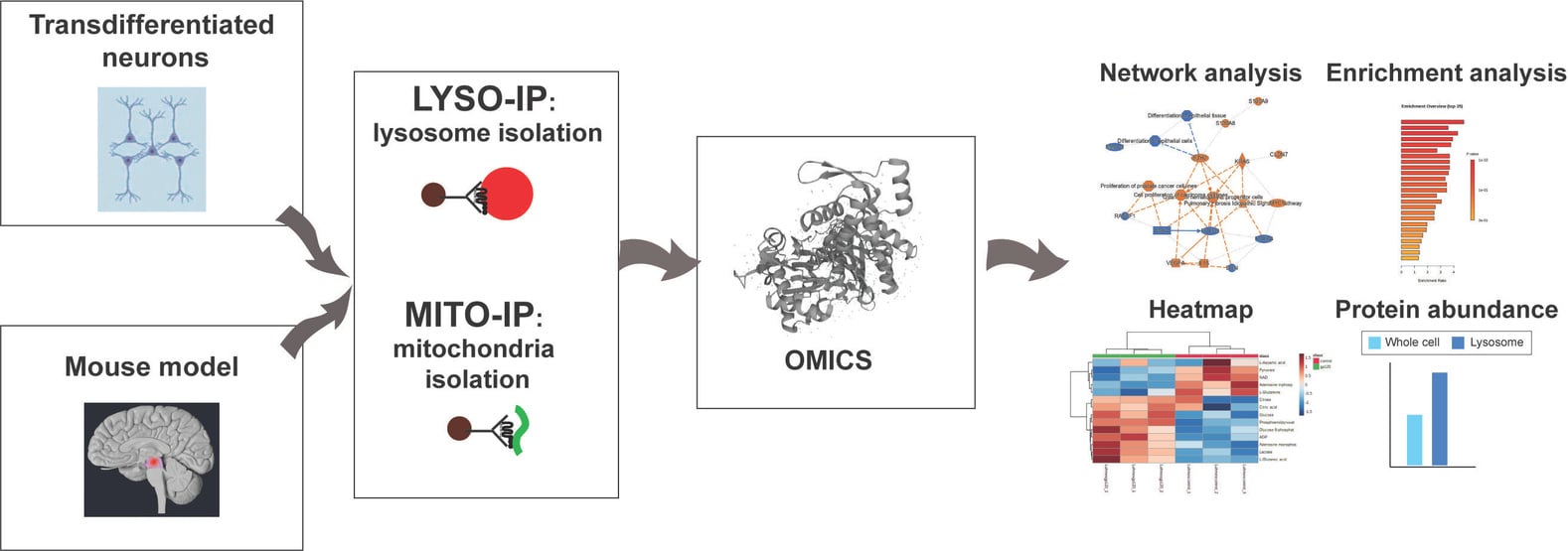
Organellar omics is a powerful, detailed, and high-resolution view of dynamic changes within specific cellular compartments, like lysosomes and mitochondria, allowing to pinpoint where and how disruptions occur. This approach enables the identification of targeted pathways and molecular mechanisms, offering more precise therapeutic strategies for diseases like neuroHIV.
PUBLICATIONS
Shrestha J, Santerre M, Allen CN, Arjona SP, Hooper R, Mukerjee R, Kaul M, Shcherbik N, Soboloff J, Sawaya BE. HIV-1 gp120 protein promotes HAND through the calcineurin pathway activation. Mitochondrion. 2023 May;70:31-40. doi: 10.1016/j.mito.2023.03.003. Epub 2023 Mar 15. PubMed PMID: 36925028; PubMed Central PMCID: PMC10484070.
Arjona SP, Allen CNS, Santerre M, Gross S, Soboloff J, Booze R, Sawaya BE. Disruption of Mitochondrial-associated ER membranes by HIV-1 tat protein contributes to premature brain aging. CNS Neurosci Ther. 2023 Jan;29(1):365-377. doi: 10.1111/cns.14011. Epub 2022 Nov 23. PubMed PMID: 36419337; PubMed Central PMCID: PMC9804058.
Shrestha J, Santerre M, Allen CNS, Arjona SP, Merali C, Mukerjee R, Chitrala KN, Park J, Bagashev A, Bui V, Eugenin EA, Merali S, Kaul M, Chin J, Sawaya BE. HIV-1 gp120 Impairs Spatial Memory Through Cyclic AMP Response Element-Binding Protein. Front Aging Neurosci. 2022;14:811481. doi: 10.3389/fnagi.2022.811481. eCollection 2022. PubMed PMID: 35615594; PubMed Central PMCID: PMC9124804.
Allen CNS, Santerre M, Arjona SP, Ghaleb LJ, Herzi M, Llewellyn MD, Shcherbik N, Sawaya BE. SARS-CoV-2 Causes Lung Inflammation through Metabolic Reprogramming and RAGE. Viruses. 2022 May 6;14(5). doi: 10.3390/v14050983. PubMed PMID: 35632725; PubMed Central PMCID: PMC9143006.
Allen CNS, Arjona SP, Santerre M, De Lucia C, Koch WJ, Sawaya BE. Metabolic Reprogramming in HIV-Associated Neurocognitive Disorders. Front Cell Neurosci. 2022;16:812887. doi: 10.3389/fncel.2022.812887. eCollection 2022. PubMed PMID: 35418836; PubMed Central PMCID: PMC8997587.
Allen CNS, Arjona SP, Santerre M, Sawaya BE. Hallmarks of Metabolic Reprogramming and Their Role in Viral Pathogenesis. Viruses. 2022 Mar 14;14(3). doi: 10.3390/v14030602. Review. PubMed PMID: 35337009; PubMed Central PMCID: PMC8955778.
Santerre M, Arjona SP, Allen CN, Callen S, Buch S, Sawaya BE. HIV-1 Vpr protein impairs lysosome clearance causing SNCA/alpha-synuclein accumulation in neurons. Autophagy. 2021 Jul;17(7):1768-1782. doi: 10.1080/15548627.2021.1915641. Epub 2021 Apr 23. PubMed PMID: 33890542; PubMed Central PMCID: PMC8354668.
Santerre M, Arjona SP, Allen CN, Shcherbik N, Sawaya BE. Why do SARS-CoV-2 NSPs rush to the ER?. J Neurol. 2021 Jun;268(6):2013-2022. doi: 10.1007/s00415-020-10197-8. Epub 2020 Sep 1. Review. PubMed PMID: 32870373; PubMed Central PMCID: PMC7461160.
Haouzi P, McCann M, Wang J, Zhang XQ, Song J, Sariyer I, Langford D, Santerre M, Tubbs N, Haouzi-Judenherc A, Cheung JY. Antidotal effects of methylene blue against cyanide neurological toxicity: in vivo and in vitro studies. Ann N Y Acad Sci. 2020 Nov;1479(1):108-121. doi: 10.1111/nyas.14353. Epub 2020 May 6. PubMed PMID: 32374444; PubMed Central PMCID: PMC7644644.
Santerre M, Bagashev A, Gorecki L, Lysek KZ, Wang Y, Shrestha J, Del Carpio-Cano F, Mukerjee R, Sawaya BE. HIV-1 Tat protein promotes neuronal dysregulation by inhibiting E2F transcription factor 3 (E2F3). J Biol Chem. 2019 Mar 8;294(10):3618-3633. doi: 10.1074/jbc.RA118.003744. Epub 2018 Dec 27. PubMed PMID: 30591585; PubMed Central PMCID: PMC6416426.
Santerre M, Chatila W, Wang Y, Mukerjee R, Sawaya BE. HIV-1 Nef promotes cell proliferation and microRNA dysregulation in lung cells. Cell Cycle. 2019 Jan;18(2):130-142. doi: 10.1080/15384101.2018.1557487. Epub 2019 Jan 6. PubMed PMID: 30563405; PubMed Central PMCID: PMC6343720.
Santerre M, Wang Y, Arjona S, Allen C, Sawaya BE. Differential Contribution of HIV-1 Subtypes B and C to Neurological Disorders: Mechanisms and Possible Treatments. AIDS Rev. 2019;21(2):76-83. doi: 10.24875/AIDSRev.19000051. Review. PubMed PMID: 31332398; PubMed Central PMCID: PMC7219600.
Wang Y, Santerre M, Tempera I, Martin K, Mukerjee R, Sawaya BE. HIV-1 Vpr disrupts mitochondria axonal transport and accelerates neuronal aging. Neuropharmacology. 2017 May 1;117:364-375. doi: 10.1016/j.neuropharm.2017.02.008. Epub 2017 Feb 14. PubMed PMID: 28212984; PubMed Central PMCID: PMC5397298.
Grove M, Kim H, Santerre M, Krupka AJ, Han SB, Zhai J, Cho JY, Park R, Harris M, Kim S, Sawaya BE, Kang SH, Barbe MF, Cho SH, Lemay MA, Son YJ. YAP/TAZ initiate and maintain Schwann cell myelination. Elife. 2017 Jan 26;6. doi: 10.7554/eLife.20982. PubMed PMID: 28124973; PubMed Central PMCID: PMC5287714.
select
Bagashev A, Mukerjee R, Santerre M, Del Carpio-Cano FE, Shrestha J, Wang Y, He JJ, Sawaya BE. Involvement of miR-196a in HIV-associated neurocognitive disorders. Apoptosis. 2014 Aug;19(8):1202-14. doi: 10.1007/s10495-014-1003-2. PubMed PMID: 24872081.
Chatila WM, Criner GJ, Hancock WW, Akimova T, Moldover B, Chang JK, Cornwell W, Santerre M, Rogers TJ. Blunted expression of miR-199a-5p in regulatory T cells of patients with chronic obstructive pulmonary disease compared to unaffected smokers. Clin Exp Immunol. 2014 Jul;177(1):341-52. doi: 10.1111/cei.12325. PubMed PMID: 24634990; PubMed Central PMCID: PMC4089184.
Marchand V, Santerre M, Aigueperse C, Fouillen L, Saliou JM, Van Dorsselaer A, Sanglier-Cianférani S, Branlant C, Motorin Y. Identification of protein partners of the human immunodeficiency virus 1 tat/rev exon 3 leads to the discovery of a new HIV-1 splicing regulator, protein hnRNP K. RNA Biol. 2011 Mar-Apr;8(2):325-42. doi: 10.4161/rna.8.2.13984. Epub 2011 Mar 1. PubMed PMID: 21368586.
Contact us!
Address:
3307 North Broad Street
Room 304
Philadelphia, PA 19140
maryline.santerre@temple.edu

